Extending the duration of use of in-line IV filters up to 96 hours
SUMMARY
Studies with uncharged 0.22 µm in-line IV filters showed bacteria were retained, but endotoxins passed through after 24–48 hours, leading to the recommendation of 24-hour filter changes . Positively charged membrane-based in-line IV filters have been validated to retain bacteria and endotoxins for up to 96 hours, enabling fewer line manipulations, reduced contamination risk, and significant time and cost savings.
Can in-line IV filters be safely used beyond 24 hours to save time and reduce costs?
Many solutions are administered for periods longer than 24 hours. Should a clinical practitioner be concerned that in-line IV filters are safe regarding endotoxin?
Yes. An in vitro study by Holmes et al simulated growth of bacteria during simulated infusion and investigated uncharged 0.22 µm in-line IV filters in respect to their retention capabilities of bacteria (R. agglomerans, S.marcescens, K. pneumoniae, and P. aeruginosa) and their associated endotoxin over a 72-hour period.1
The study showed that whilst the gram-negative bacteria proliferated in the solutions upstream of the in-line IV filters, none of the 0.22 µm in-line IV filters allowed the passage of the bacteria downstream over the 72-hour period. However, for all 4 of the gram-negative bacteria used endotoxin was detected downstream of the in-line IV filters between 24 and 48 hours and led to the conclusion that “to avoid this potential hazard of terminal filtration, in-line filter sets should be changed every 24 hours.”
On the downside, changing in-line IV filter every 24 hour leads to substantial cost, staff time and set manipulations with associated risks of bacterial contamination.2-7
Extending the usage time of the in-line IV administration set with an endotoxin-retentive filter can lead to substantial cost and time savings and potentially minimizes the risk of bacterial contamination due to less manipulation of the IV lines.8
Extending the duration of use of in-line IV filters up to 96 hours
It would be reasonable to expect that any in-line IV filter intended for use for more than 24 hours in an infusion line would be validated, to show that it retains endotoxin in infusion lines. Several studies have evaluated the endotoxin-retention properties of 0.2 µm filters during simulated clinical infusions. Those studies demonstrated that, distinct differences exist in the ability to retain endotoxins under those test conditions.9-12
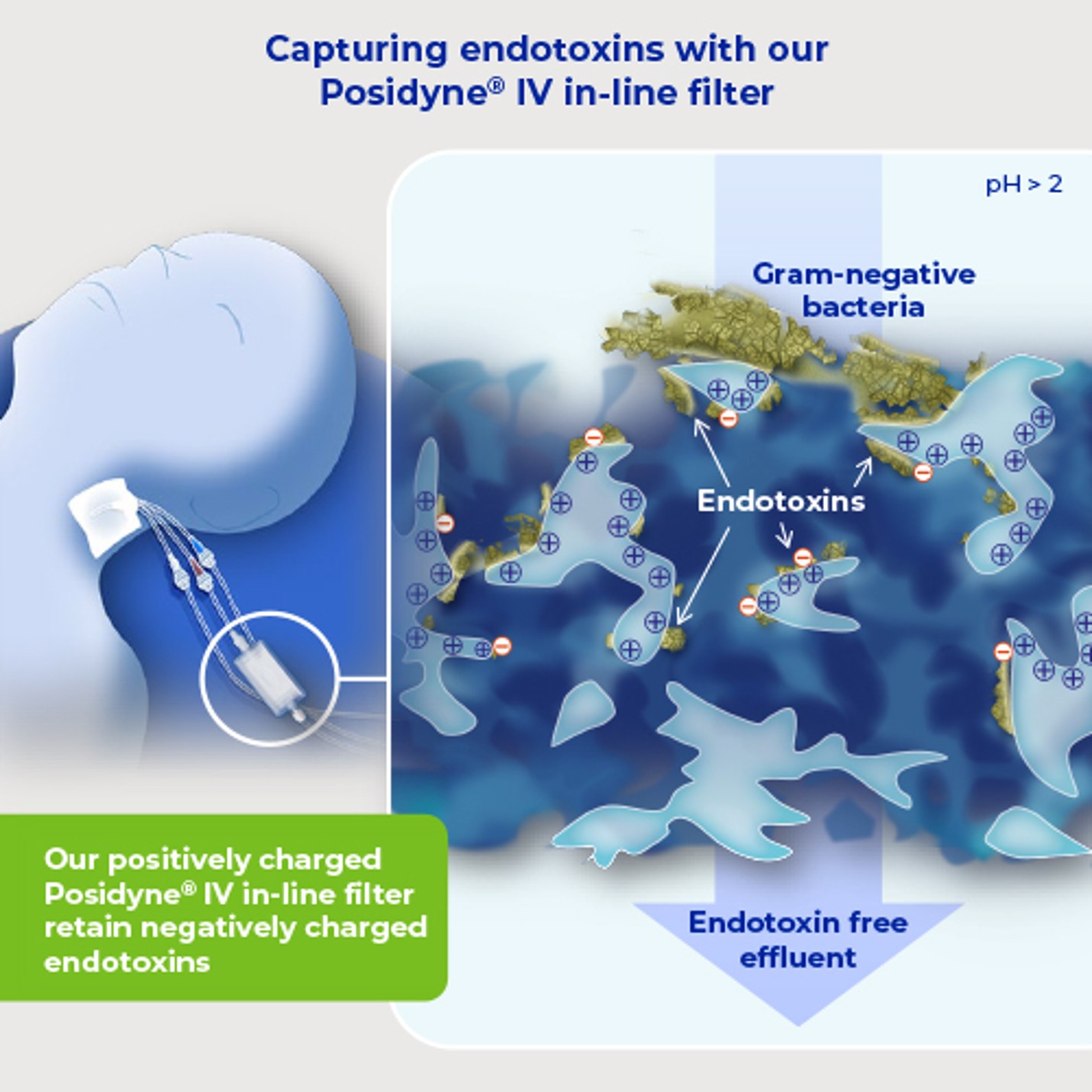
We have manufactured positively charged, membrane-based intravenous filter products with a claim for the removal of bacteria and associated endotoxins up to 96 hours for the health care community for over four decades.
The endotoxin aggregate shed from the cell is a particle with a high negative charge. It is possible to retain these aggregates by the incorporation of a positive charge, at an appropriate density and configuration, in the filter membrane. The addition of a positive charge does not automatically guarantee reliable endotoxin retention and extensive testing is necessary.
Endotoxin contains exposed phosphate groups. Generally, at pH values above pH 2, these phosphate groups are strongly negatively charged. IV solutions have a pH value above this and our positively charged in-line IV filter therefore provide the opportunity for the removal of the negatively charged endotoxins.
Explore our IV endotoxins removal solutions
References
1.Holmes C.J. et al. (1980). Potential Hazards Associated with Microbial Contamination of In-Line Filters During Intravenous Therapy. Journal of Clinical Microbiology; 12 (6): 725-731
2.Stromberg G, Wahlgren J. (1989). Saving money with effective inline filters. Intens Care Nurs; 5 (109)
3.Barber N, Jacklin A. (1987). CCU drug costs—the pharmacists’ role. Int Care World; 4 (80)
4.Ballard K. (1990)Showing where the money goes: cost-effective care in ICU. Prof Nurse: 565
5.Clarke R. (1990). A cost-effective system for TPN. Nurs Times; 86: 65
6.Puntis JWL, Booth IW. (1990). The place of a nutritional care team in paediatric parctice. Intens Ther Clin Monitor; 11 (132)
7.Cousins D. (1988). Cost savings in IV therapy. Care Crit Ill; 4 (1)
8.Bethune K. et al. (2001). British Pharmaceutical Nutrition Group Working Party. Use of filters during the preparation and administration of parenteral nutrition: position paper and guidelines prepared by a British pharmaceutical nutrition group working party. Nutrition; 17 (5): 403-8
9.Baumgartner, T. G. et al. (1986). Bacterial endotoxin retention by inline intravenous filters. Am. J. Hosp. Pharm; 43:681-684
10.Horibe, K. et al. (1990). Evaluation of the endotoxin retention capabilities of inline intravenous filters. JPEN J. Parenter. Enteral. Nutr; 14: 56-59
11.Richards, C. & Grassby P. F. (1994). A comparison of the endotoxin-retentive abilities of two ‘96-h’ in-line intravenous filters. J. Clin. Pharm. Ther; 19 (3): 199-202
12.Spielberg, R. & J. Martin. (1985). Evaluation of the endotoxin/bacterial retention of I.V. filters during simulated extended infusions, p. 1001. In Technical note IV. Pall Biomedical Ltd., Portsmouth, United Kingdom.
Author bio
Dr. Luibl is a Sr. Marketing Manager Medical Content with knowledge in medical device and clinical science.
